
73
Cannabinoids in seed extracts of Cannabis sativa cultivars
H. Mölleken1 and H. Husmann2
1Bergische Universität, Physiologische Chemie der Pflanzen, Gaußstraße. 20, 42119 Wuppertal, Germany
2Max-Planck-Institut für Kohlenforschung, Abt. Chromatographie, Kaiser-Wilhelm-Platz 1, 45470 Mülheim/Ruhr, Germany
Mölleken, H. and H. Husmann 1997. Cannabinoids in seed extracts of Cannabis sativa cultivars. Journal of the International Hemp Association 4(2): 73, 76-79. Cannabinoids are of great interest for pharmaceutical and medical purposes. However, as some of them are also used as recreational drugs, forensic and economic problems could result if they occur in food products made from hemp seeds with cannabinoid concentrations that are high enough to have a psychoactive effect. We have therefore (a) developed a method to determine cannabinoids in microgram quantities in every hemp product or in any part of the plant within 15-30-minutes analysis time and (b) analyzed various hemp oils and plant extracts of Cannabis cultivars from the EU and other regions to determine their cannabinoid content. Our investigations have proven that the active substance cannot be found inside the achene, but may be attached to the shell (pericarp) of the fruit. Thus, the content of THC in hemp oil can come only as a result of the technical process of harvesting the fruits.

Cannabis fruits show great variability both in their morphological and chemical characteristics.
Introduction
Many chromatographic methods have been applied to the
forensic detection of cannabinoids, especially Δ9-tetrahydrocannabinol
(THC), in media such as blood and urine. Rarely, however, have researchers been
concerned with the cannabinoids in hemp oil manufactured for nutritional
purposes (Alt and Reinhardt 1996, Brenneisen 1984, Callaway et al. 1997,
Lanyon et al. 1981, Law et al. 1984, McBurney et al. 1986,
Meesters and Eggink 1996, Norman et al. 1971, Novotny et al. 1976,
Paul et al. 1987, Nakamura et al. 1990, Pertwee 1997, Struemper et
al. 1997, Vicki et al. 1973, Vree 1972, Vree et al. 1973,
Whiting and Manders 1982).
We have
thus developed a method to analyze cannabinoids in hemp oil. This new method can
be used either directly, for analysis of an oil dissolved in methylene chloride,
or indirectly by extraction of the oil or the fruits in methanol, whereby the
methanol supernatant is analyzed after sedimentation of the oil phase or of the
fruits. An advantage to this method is that after derivatization, each
methylated cannabinoid (CB-OME), and each fatty acid (FA) as its fatty acid
methyl ester (FAME), can be determined in a single GC analysis. It allows a
quick and precise determination of Δ8-THC,
Δ9-THC,
cannabinol (CBN), cannabidiol (CBD) and most probably, other cannabinoids we
have not investigated. We have used this method to look for cannabinoids in
various hemp oils, in extracts of the “seeded“ flowers and in parts of these
fruits.
Materials and Methods
Various Cannabis strains including industrial hemp
cultivars from the European Union and Eastern Europe were analyzed. Hemp fruits
(1 g), either with or without removal of adherent particles through agitation in
methanol, were homogenized in methanol, then treated with trimethylsulfonium
hydroxide (TMSH) to produce FAMEs from the FA residues of the triglycerides (Mölleken
and Theimer 1997 a, b) and methyl ethers of the cannabinoids. Hemp oils and
various parts of the plant were directly dissolved in methanol before
methylation as described previously.
The
resulting CB-OMEs and FAMEs were analyzed on an HP 5890 gas chromatograph (GC)
equipped with either an FID or an HP 5870 Mass Selective Detector (GC-MS). OV-1
columns were used.
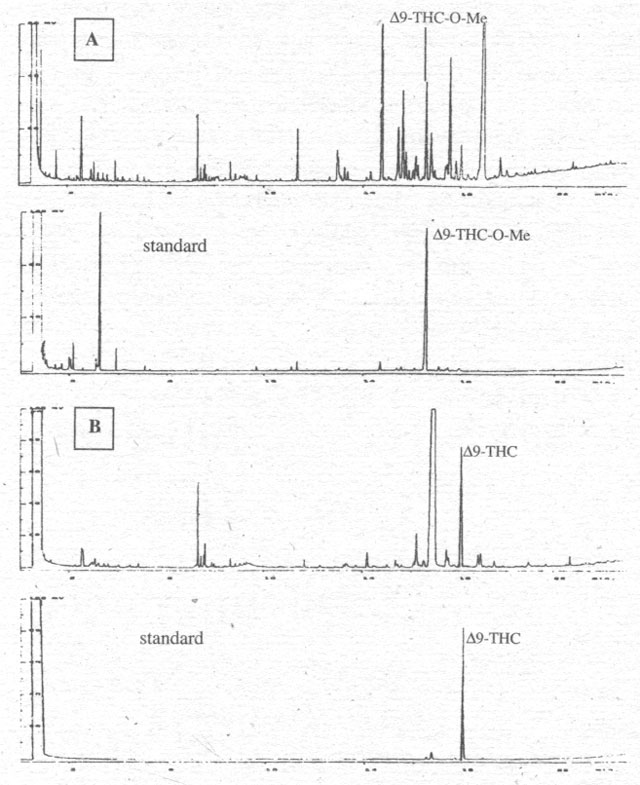
Figure 1. Chromatograms with A: indirect detection of Δ9-THC as Δ9-THC-O-Me after derivitisation with TMSH in methanol of the cannabinoid of the hemp oil from C. sativa var. Alice; B: direct detection of Δ9-THC after extraction with methanol of the hemp oil from C. sativa var. Alice.
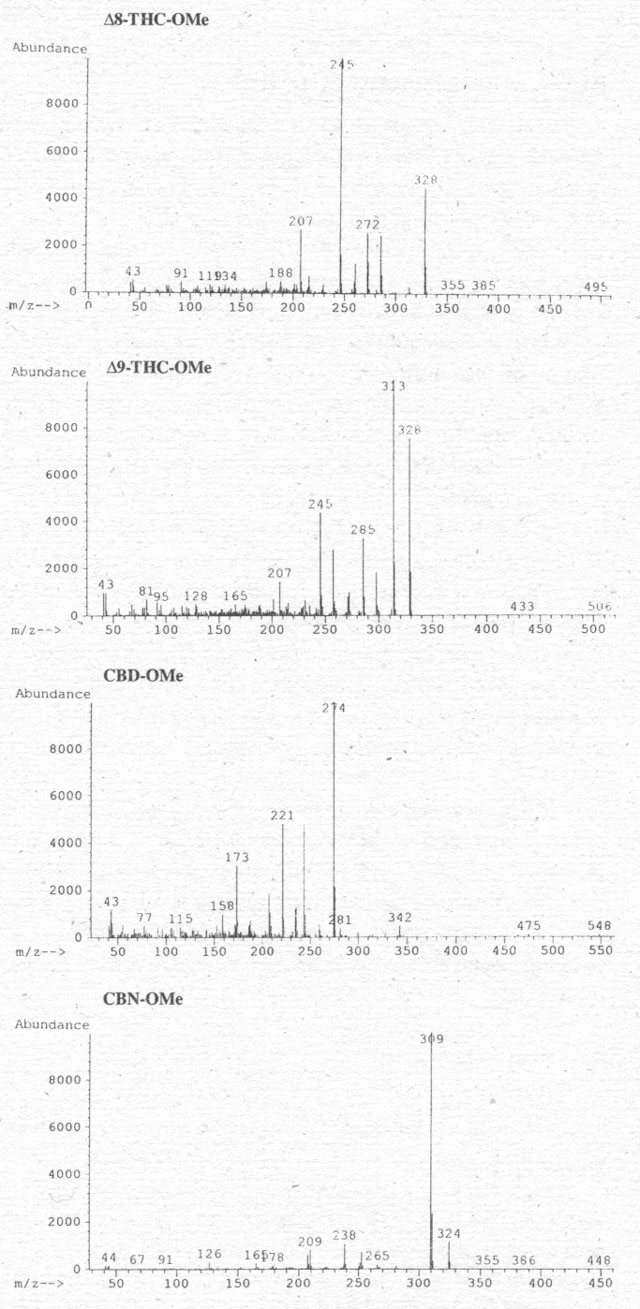
Figure 2. Mass spectra of the four cannabinoids after methylation with TMSH in methanol.
Results
Methodological Approach
Figure 1 compares the direct and indirect methods for
detecting Δ9-THC
in the oil of one hemp variety. It becomes clear that both analyses are
successful, though the retention times (RT) are shifted, with the methylated
substances being detected earlier. The methylation seems to result in better
resolution, however, so that monoterpenes and sesquiterpenes in flower extracts
and FAMEs in hemp oils can be determined at the same time.
As the
above analyses characterized only Δ9-THC,
we undertook a second experiment in which we looked for the methylation of other
cannabinoids such as Δ8-THC,
CBD and CBN, as well. We first examined a mixture of these substances by GC-MS
(Fig. 2) after methylation (compare Pfleger et al. 1993). It is clear
that (a) all cannabinoids are methylated and (b) the molecular ion of CBD shows
that both hydroxy groups are methylated. Further details of the GC-conditions
may be published after testing the method on urine and blood specimens after
ingestion of (a) hemp oil and (b) hashish.
Thus, we
have found a method that allows the determination of cannabinoids alongside any
triglyceride (TG) in the sample. It is also possible to determine THC directly,
without derivatisation of the hemp oil. However, the high-boiling TGs cannot be
eliminated from the column under the chromatographic conditions used for the
detection of cannabinoids, and so in the course of time, will accumulate on the
column, hindering the precision of subsequent analyses.
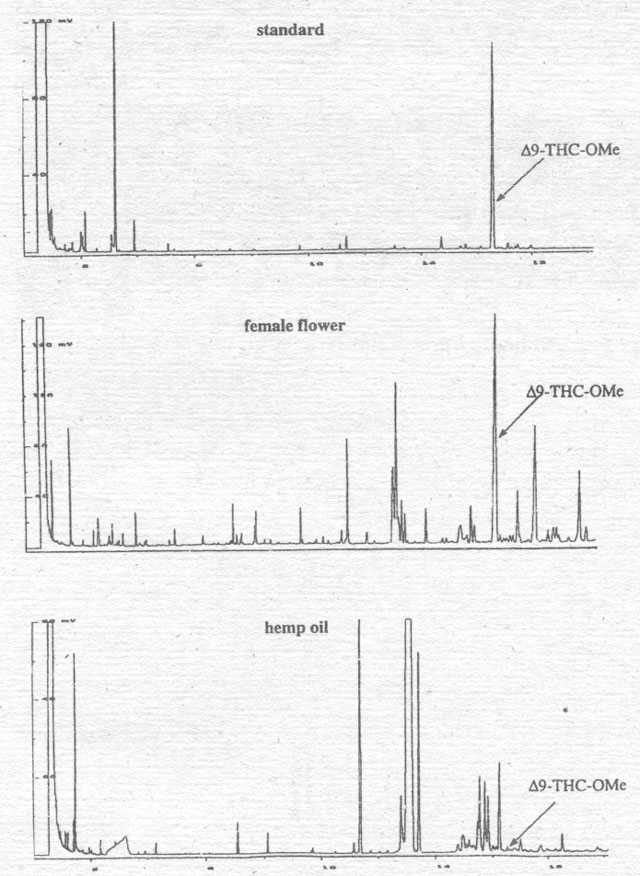
Figure 3. Comparison of the hemp oil extraction from the fruits with the female flower of the Hungarian variety Alice.
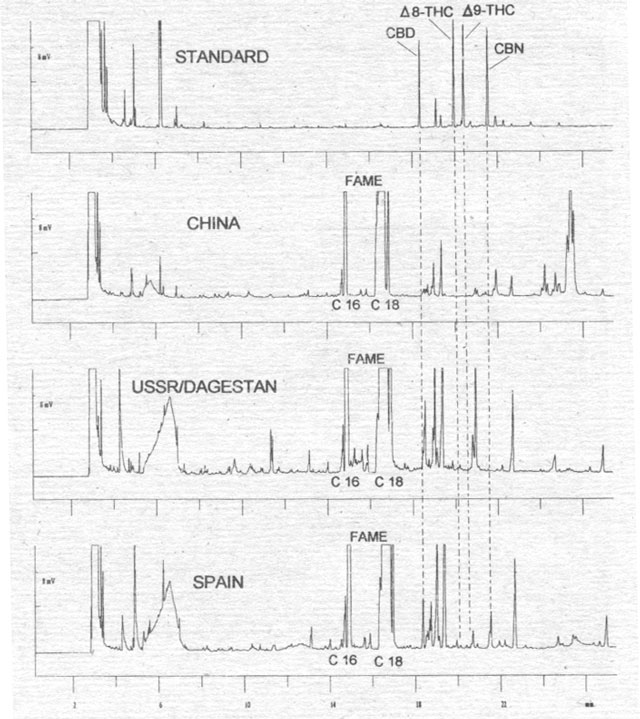
Figure 4. Analysis of hemp fruit extracts (without cleaning the shell) from Chinese, USSR/Dagestan and Spanish varieties for cannabinoids as CB-OMe, derivitisation with TMSH. FAME = fatty acid methyl ester.
Cannabinoids in hemp oil and fruit extracts
First we compared the Δ9-THC
in a hemp oil extracted from the fruits of the Hungarian variety “Alice“ with
that found in its female flower (Fig. 3). Whereas the flower extract shows a
peak like that of the Δ9-THC-standard,
the “seed“ oil does not contain any Δ9-THC.
To
verify this analysis, we selected fruits from varieties of several origins (i.e.,
China, Dagestan, Spain) whose dried flowers normally contain 2-4 % Δ9-THC
by dry weight (VIR 1977). We analyzed the effect of the fruit extraction method
on cannabinoid content (Fig. 4). Whereas extracts from the complete fruits of
the Chinese and Russian (Dagestan) varieties do not show any cannabinoid
content, the Spanish variety shows large peaks for CBD and CBN. We therefore
agitated fruits of this hemp variety with methanol for a few minutes to remove
adherent particles (Fig. 5). The analyses of the clean fruits confirm that there
are no cannabinoids in the seed. The only opportunity for cannabinoids to enter
the oil is in the course of the harvest or other technical processes. These
viscous substances are bound in the outer wax layer of the shell (pericarp), and
they could enter the oil when it is being pressed (compare with Bósca 1995).
To
support this interpretation, we have compared the analyses for cannabinoids of
oil extracts of the fruits (Fig. 6) and various hemp oils (Fig. 7) with a
cannabinoid standard. Oils from varieties of the EU do not contain cannabinoids
(for example Félina 34), with the exception of one oil from a mixture of
several hemp varieties (Fédora/Futura), which shows a small peak representing
CBN in picogram quantities/sample. Only one oil, from Hungary, contains all
three cannabinoids in concentrations that hint at microgram quantities/sample.
Discussion and Conclusion
In general, Cannabis contains three major
cannabinoids, CBD, CBN, and Δ9-THC
(Alt and Reinhardt 1996, Brenneisen 1984, Turner et al. 1980, Vree et
al. 1971, 1972) that require an unambiguous detection in hemp oil for
forensic reasons. Therefore, to analyze the cannabinoids in hemp oil, we
established a method that allows a quick, but very precise determination,
whereby these three compounds are well separated. The methylation guarantees a
precise determination and at the same time, the triglycerides do not accumulate
on the column. If desired, the FAMEs can be determined in the same analysis.
Thus, this chromatographic method is sufficiently simple and reproducible to be
used for applications of forensic interest. As Nakamura et al. (1990)
established a comparable analysis for the Δ9-THC-metabolite
(11-nor-Δ9-THC-COOH
in urine), we will test this method on urine and blood samples in the future.
With
this method, we are not only able to identify the psychoactive cannabinoids, but
we also get many peaks representing such cannabinoids, as cannabichromene or
cannabigerol. Furthermore, Δ8-THC may
be used as an internal standard in the analysis of hemp oils for cannabinoids,
since it is not naturally occurring.
Up to
now, we have analyzed only Δ9-THC
and three additional cannabinoids, as we have no other standards for comparison.
We are, however, confident that all the cannabinoids can also be detected by
this method. Others have already established methods that give good analyses,
but these methods have not been tested for hemp oil analyses and require more
time for measurement and preparation of the sample (Alt and Reinhardt 1996,
Brenneisen 1984, Lanyon et al. 1981, Law et al. 1984, Mc Burney et
al. 1986, Doorenbos et al. 1971, Meesters and Eggink 1996, Norman et
al. 1971, Novotny et al. 1976, Paul et al. 1987, Nakamura et
al. 1990, Pertwee 1997, Struemper 1997, Vicki et al. 1973, Vree et
al. 1972, Vree et al. 1973, Whiting and Manders 1982).
There
have been only a few analyses of cannabinoids in hemp oil (Alt and Reinhardt
1996, Brenneisen 1995, Máthé and Bócsa 1995). Máthé and Bócsa analyzed
only one variety, ‘Kompolti’, and Alt and Reinhardt made tests of urine and
blood from test subjects that ate various hemp oils.
These
analyses prove by detailed investigations that hemp fruits do not contain any
cannabinoids. Though Doorenbos et al. (1971) describe a cannabinoid
content of plant parts decreasing in the following order: bracts, flowers,
leaves, small stems, large stems, roots and seed, we could not find an
indication of cannabinoids in the hemp fruit varieties we analyzed. Fetterman et
al. (1971) also found traces of CBD, THC and CBN in some varieties after
cleaning the fruits with chloroform. Vogelmann (1988) found to the contrary,
that cannabinoids seem to appear first in the seedlings, cannabichromene being
the first cannabinoid that can be detected.
The
opinion that hemp oil contains cannabinoids (Alt et al. 1996, Struempler et
al. 1997) must be qualified. Hemp oils from fruits with cleaned shells
should not contain Δ9-THC.
Other oils may have low to high concentrations. An industrial technique that
separates the shell from the fruits would make it possible to produce a hemp oil
without any cannabinoids.
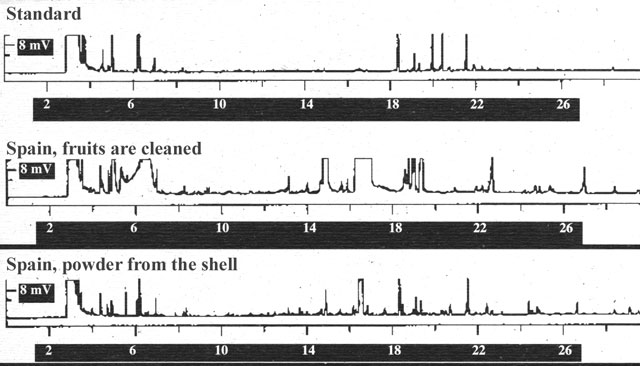
Figure 5. Analysis of particles washed from the shell of the Spanish hemp variety (derivitisation of the cannabinoids to CB-OME with TMSH in methanol).
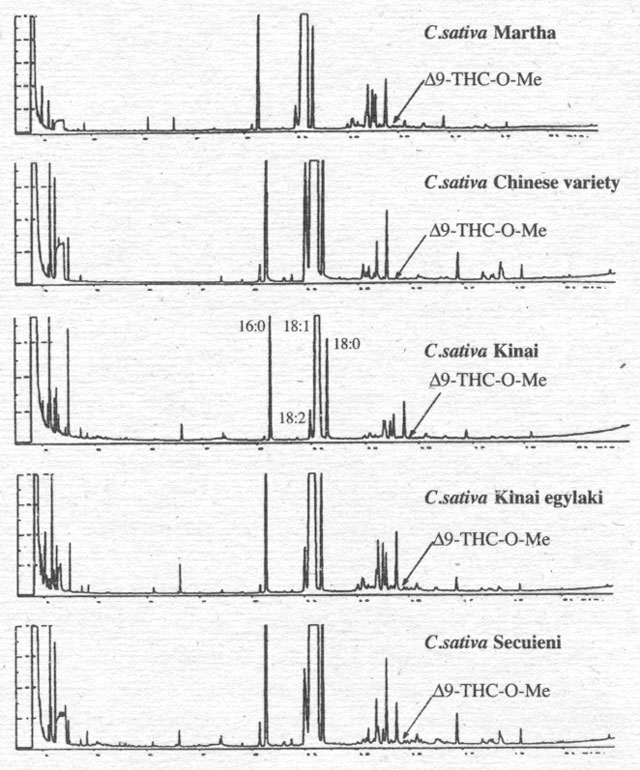
Figure 6. Analysis of Δ9-THC-O-Me in oil extracts of fruits of some Cannabis varieties (fruits are pressed in methanol and derivitised with TMSH).
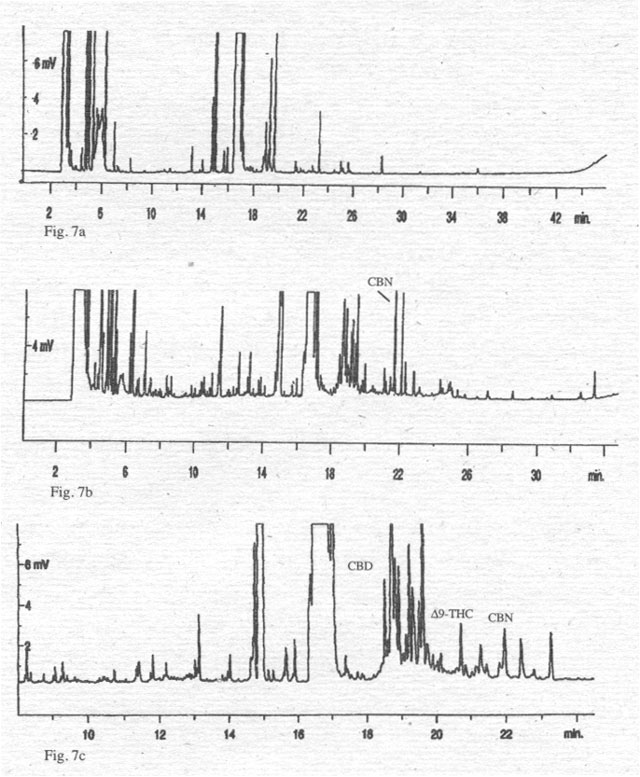
Figure 7. Chromatograms of cold pressed hemp oils (100 ml oil/Methanol extract 2:1 + 200 ml TMSH). 7a: hemp oil from ‘Felina 34’; 7b: hemp oil from a mixture of ‘Fedora’/’Futura’; 7c: hemp oil from a Hungarian variety.
References