65
Decimal code for growth stages of hemp (Cannabis sativa L.)
Vito Mediavilla1, Manuel Jonquera1, Ingrid Schmid-Slembrouck2 and Alberto Soldati2
1 Swiss Federal Research Station for Agroecology and Agriculture (FAL), Reckenholzstrasse 191, CH-8046 Zurich, Switzerland; E-mail: vito.mediavilla@fal.admin.ch
2 Swiss Federal Institute of Technology, Department of Agriculture and Food Science, Institute of Plant Sciences, CH-8315 Lindau, Switzerland
Mediavilla, Vito, Manuel Jonquera, Ingrid Schmid-Slembrouck and Alberto Soldati 1998. A decimal code for growth stages of hemp (Cannabis sativa L.). Journal of the International Hemp Association 5(2): 65, 68-74. A decimal code for hemp (Cannabis sativa L.) growth stages is presented. The life cycle is divided into four principal stages: germination and emergence, vegetation, flowering and seed formation and senescence. Each principal stage is subdivided into secondary stages. The practical use of the code is discussed with particular reference to different yield components.
Introduction
A standardized scale for recording the growth stages of a crop is clearly useful
for agronomists, physiologists, pathologists, breeders and, of course, for
farmers. Codes for development of important crops like maize, cereals, rape,
pea, faba bean and potato have been described (Hanway 1963, Zadoks et al.
1974, Sylvester-Bradley et al. 1984, Knott 1987, Knott 1990 and Jefferies
and Lawson 1991).
Although
many authors (e.g., Ceapoiu 1958, de Meijer et al. 1992,
Slembrouck 1994, van der Werf et al. 1995a, Bócsa and Karus 1997, Clarke
1997, von Buttlar et al. 1997) have described the characteristics of
growth and development of hemp (Cannabis sativa L.), little attention has
been given to a formal assessment and recording of these stages.
Material and Methods
The general principles for a decimal code proposed by Zadoks et al.
(1974) for cereals have been adjusted for hemp.
| First-digit of code | Definition | |
|---|---|---|
| 0 | Germination and emergence | |
| 1 | Vegetative stage | |
| 2 | Flowering and seed formation | |
| 3 | Senescence | |
Principal growth stages
Four
principal stages describe the life cycle of a plant (Table 1) and are coded by
their first digit of a four-digit code.
| Code | Definition | Remarks | |
|---|---|---|---|
| Germination and emergence | |||
| 0000 | Dry seed | ||
| 0001 | Radicle apparent | ||
| 0002 | Emergence of hypocotyl | ||
| 0003 | Cotyledons unfolded | ||
| Vegetative stage refers to main stem. Leaves are considered as unfolded when leaflets are at least one cm long. | |||
| 1002 | 1st leaf pair | 1 leaflet | |
| 1004 | 2nd leaf pair | 3 leaflets | |
| 1006 | 3rd leaf pair | 5 leaflets | |
| 1008 | 4th leaf pair | 7 leaflets | |
| 1010 | 5th leaf pair | ||
| : | : | ||
| 10xx | 11th leaf pair | xx = 2(nth leaf pair) | |
| Flowering and seed formation refers to the main stem including branches. | |||
| 2000 | GV point | Change of phyllotaxis on the main stem from opposite to alternate. Distance between petioles of alternate leaves at least 0.5 cm |
|
| 2001 | Flower primordia | Sex nearly indistinguishable | |
| Dioecious Plant | |||
| male | |||
| 2100 | Flower formation | First closed staminate flowers | |
| 2101 | Beginning of flowering | First opened staminate flowers | |
| 2102 | Flowering | 50% opened staminate flowers | |
| 2103 | End of flowering | 95% of staminate flowers open or withered | |
| female | |||
| 2200 | Flower formation | First pistillate flowers Bract with no styles |
|
| 2201 | Beginning of flowering | Styles on first female flowers | |
| 2202 | Flowering | 50% of bracts formed | |
| 2203 | Beginning of see maturity | First seeds hard | |
| 2204 | Seed maturity | 50% of seeds hard | |
| 2205 | End of seed maturity | 95% of seeds hard or shattered | |
| Monoecious Plant | |||
| 2300 | Female flower formation | First pistillate flowers Perigonal bracts with no styles |
|
| 2301 | Beginning of female flowering | First styles visible | |
| 2302 | Female flowering | 50% of bracts formed | |
| 2303 | Male flower formation | First closed staminate flowers | |
| 2304 | Male flowering | Most staminate flowers open | |
| 2305 | Beginning of seed maturity | First seeds hard | |
| 2306 | Seed maturity | 50% of seed hard | |
| 2307 | End of seed maturity | 95% of seeds hard or shattered | |
| Senescence | |||
| 3001 | Leaf desiccation | Leaves dry | |
| 3002 | Stem desiccation | Leaves dropped | |
| 3003 | Stem decomposition | Bast fibers free | |
Secondary growth stages
These stages are described (Table 2) by the second digit,
which indicates the sex of the plant, the third and fourth digits, which
indicate the developmental stage of the plant.

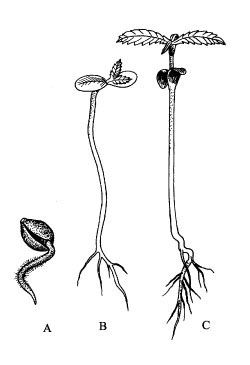
A - radicle apparent (code 0001), B - cotyledons unfolded (code 0003), C - first leaf pair (code 1002).Figure 1.
Germination and emergence (principal stage code 0)
After water intake (imbibition), the radicle appears (Figure
1A), the hypocotyl emerges and the cotyledons unfold above the soil (Figure 1B).
The optimal temperature for germination is 24º C (Ceapoiu 1958), lower
temperatures delay the emergence. The minimum temperature for germination is 0º
C (van der Werf et al. 1995a). Germination usually takes three to seven
days (Clarke 1997). In contrast to the subsequent leaves, cotyledons are sessile
and have no serrate margin.
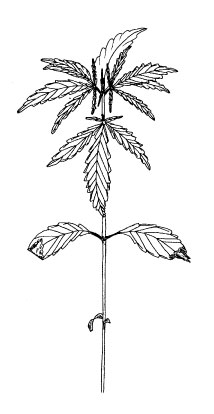
Third leaf pair (code 1006).Figure 2.
Vegetative stage (principal stage code 1)
This stage is defined as the stage between emergence and
generative development and is characterized by the growth of the stem and
leaves. At the beginning of the vegetative stage, the plant grows slowly. In
this phase, the plant usually forms up to five true leaf pairs and short
internodes (Figure 2). Later, during the fast stem elongation, internodes are
longer (Ceapoiu 1958, Bócsa and Karus 1997). During the whole vegetative stage,
usually seven to twelve leaf pairs are formed. The first leaf pair consists of a
single leaflet (Figures 1C and 2). The second leaf pair has three leaflets, the
third leaf pair has five leaflets and so on, usually up to eleven leaflets
(Clarke 1997). We consider a leaf as unfolded when its leaflets are at least one
cm long.
Vegetative stage is defined by the number of fully developed
leaves. Code 1002 is used for the first leaf pair, code 1004 for the second leaf
pair and code 10xx for the nth leaf pair (xx = 2n). If the lower leaves have
already been shed, it is necessary to count the nodes, taking into consideration
that one node carries two leaves and that the first node belongs to the
cotyledons.
Fiber hemp stems are usually not branched, but the stem can
be ramified at low plant density. However, for the characterization of the
growth of hemp plants, branches are not taken into account for coding during the
vegetative stage.
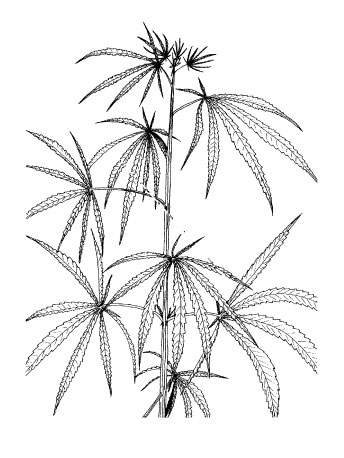
Change of phyllotaxis (code 2000).Figure 3.
Flowering and seed formation (principal stage
code 2)
Depending on cultivar and day length, the change of
phyllotaxis (leaf position) from opposite to alternate (Figure 3) indicates the
induction of flowering (or "GV point"; Bócsa and Karus 1997). The
first, nearly indistinguishable, flower primordia may appear and care has to be
taken that they are not mistaken for branch primordia. Male primordia can be
identified by their curved claw shape, soon followed by the differentiation of
round pointed flower buds having five radial tepal segments. The females are
recognized by the enlargement of a symmetrically tubular perigonal bract
(incorrectly called calyx by Clarke 1997). Because this identification is
difficult, sex differentiation of primordia (Figure 4) is not considered by the
coding system. In this phase, stem elongation slows down. The appearance of
flower primordia, as well as the process of flowering, proceeds from the base
upwards to the top of the inflorescence (Clarke 1997).
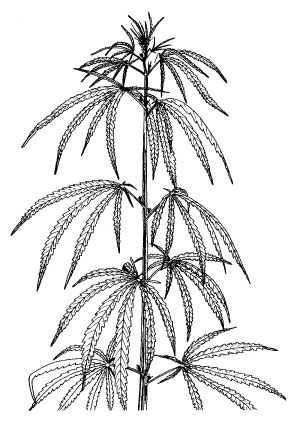
Flower primordia (code 2001).Figure 4.
After the GV point (code 2000) and the appearance of flower primordia (code 2001) have been reached, we distinguish the generative phase of male, female and monoecious plants by the second digit: ‘1’ for male, ‘2’ for female and ‘3’ for monoecious plants. The third and fourth digits refer to the exact generative stage. Reproductive organs on branches are taken into consideration for the description of flowering and seed formation.
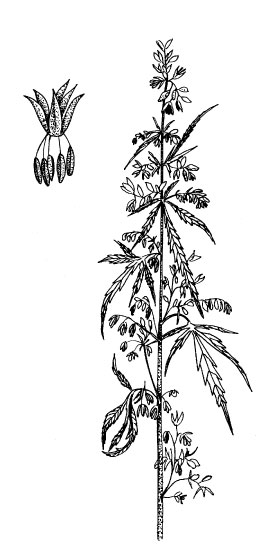
A: Staminate flowers open (code 2102), B: detail of a staminate male flower.Figure 5.
On dioecious male plants, staminate flowers usually appear about two weeks before the styles of female plants (Clarke 1997). The male inflorescence is branched and the hundreds of individual male flowers are in different stages of floral maturation (Figures 5. A-B). We define flower formation (code 2100) as the appearance of the first still closed male flowers and the beginning of flowering (code 2101) as when the first staminate flowers are open. The peak of the male flowering stage is reached when about 50% of the staminate flowers of one plant are open and pollen is released (code 2102). The end of flowering (code 2103) is reached when 90% of all the flowers are, or have been, opened.
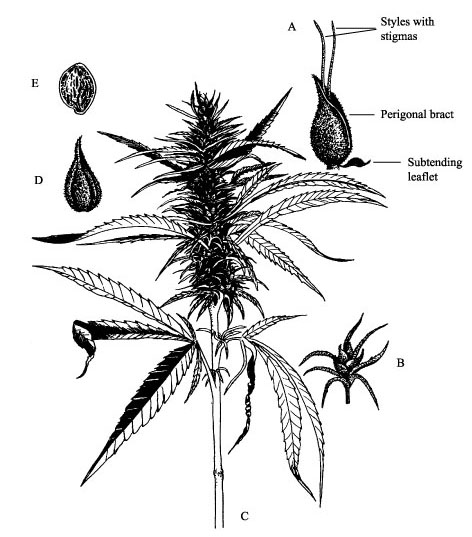
A: Pistillate female flower (stigmas, style, perigonal bract and stipule), B: spike, C: inflorescence, D: formed perigonal bract, E: hard seed.Figure 6.
On dioecious female plants, the appearance of the first
perigonal bracts with no styles indicates that the female inflorescences will
soon flower (code 2200). The inflorescence of female plants is leafy and
compact. The female flower is hidden within the perigonal bract and is small,
green and inconspicuous. At anthesis (pollen release) two styles protrude from
each perigonal bract (Figure 6. A). One female plant has numerous flowers in
different developmental stages (Figures 6. B-C). We define the beginning of
female flower formation (code 2201) as when the first styles are visible, and
the peak of this flowering stage is reached when about 50% of the bracts within
the inflorescence are formed, independent of whether styles are visible or not
(code 2202; Figures 6. A, D). After blooming and fertilization, seeds (achenes)
turn hard and seed shed begins (code 2203; Figure 6. E). Single seeds mature in
three to five weeks. Seed maturity is reached when 50% of the seeds are hard or
have been shed (code 2204).
On monoecious plants, male and female flowers are formed.
The ratio of male : female flowers depends both on the cultivar and on the
individual plant. Flowering stage (code 2302) refers to female flowers and is
similarly defined as for dioecious female plants. Male flowers usually appear
during female bloom (codes 2303 and 2304), on the tips of female branches. Seed
maturity is defined as for dioecious female plants (code 2306).
Senescence (principal stage code 3)
After flowering of the male, as well as after seed maturity
of female or monoecious plants, leaves and stems start to dry (codes 3001 and
3002). Due to frost in temperate regions, the plants die and decomposition of
the stem tissue causes the bast fibers to become free (code 3003).
Growth stages of a crop
Definitions of developmental stages refer to individual
plants. For agronomic purposes, however, it is necessary to be able to describe
the growth stage of a field or a population (e.g., as ‘a proportion at
stage ...’ or ‘at a range of stages ... to ...’). The morphology of Cannabis
plants varies, depending on sex and growing conditions. The proportion of
dioecious males, dioecious females and monoecious plants within a population
depends on the cultivar. In a dioecious crop, male and female plants are usually
present in about equal numbers. In a monoecious crop (e.g., French
cultivars), depending on seed production, there are usually up to 30% male
plants as well as a number of true-female plants (de Meijer 1995).
In order to identify the developmental stages of the crop, it
is necessary to determine the stage of a sample of plants taken at random from
the crop. For pea, faba bean and potato, a sample size of 20 plants is
considered to represent the crop (Knott 1987 and 1990, Jefferies and Lawson
1991). But because of sex differences and self-thinning effect (Van der Werf et
al. 1995b, I. Bócsa pers. comm. 1998), we propose a sample size of 50 to
100 plants for fiber hemp. For plot trials, the sample size can be reduced to 30
plants.
In agreement with de Meijer et al. (1992) and de
Meijer and Keizer (1994) the "limit of 50% of the plants": was chosen
for crop description (Table 3), e.g., male flowering is defined as the
stage when 50% of the male plants have reached the code 2102. Flowering of
dioecious female and monoecious is determined when 50% of plants in a population
are in the stage of codes 2202 or 2302, and seed maturity when 50% of
seed-carrying plants reached codes 2204 or 2306.
| Plant code | Crop stage | Criterion | |
|---|---|---|---|
| 0002 | Emergence | 50% of expected plants | |
| 2000 | Flower induction | 50% of all plants | |
| 2102 | Dioecious male flowering | 50% of all male plants | |
| 2202 | Dioecious female flowering | 50% of all female plants | |
| 2302 | Monoecious flowering | 50% of all monoecious plants | |
| |
2204 or 2306 |
Seed maturity |
50% of female or monoecious plants |
Practical use of a code for Cannabis growth stages
Depending on the use of the hemp crop, harvest time has an
important effect on yield and quality of hemp products. For best fiber quality,
harvest should be carried out during male flowering (code 2102) or during
flowering of monoecious crops (code 2302; Bócsa and Karus 1997). Unfortunately,
the European Union states that hemp harvesting - independent of utilization -
has to start after seed formation, i.e. code 2204 or 2306 (Hennink 1997,
European Union 1998). This stage is the best time for seed harvest (Bócsa and
Karus 1997). For the production of essential oil, the recommended stage is one
to three weeks before seed maturity (codes 2203 and 2305; Meier and Mediavilla
1998). At this time the highest level of the secondary compounds (e.g.,
volatile compounds) is reached.
Another practical use of growth stages is fertilization. In
contrast to the custom in most countries, where all fertilizer are given at
planting, Mediavilla et al. (1998), recommends for Switzerland to split
the nitrogen fertilization in order to reduce nitrogen loss. One rate is given
at emergence (code 0002) and the second rate when the third leaf pair has been
formed (code 1006).
For the determination of cannabinoid content, in accordance
with other authors, the inflorescences were collected at the beginning of the
seed maturity (code 2203 and 2305; de Meijer et al. 1992). The appearance
of diseases can be easily described by using a decimal code (McPartland 1996a,
1996b). For computer storage of data, referring to the development of plant
populations, e.g., for growth analysis, the decimal code is very useful.
Acknowledgments
The help of the International Hemp Association is gratefully
acknowledged. Part of this work was financially supported by several Cantons in
the northwest of Switzerland and the Institut Transfrontalier d’Application et
Développement Agronomique (Colmar, France).
References
Bócsa, I. and M. Karus 1997. Der Hanfanbau - Botanik, Sorten, Anbau und Ernte. [Hemp cultivation - botany, varieties and harvest.] C.F. Müller, Heidelberg, Germany. [in German]
Ceapoiu, N. 1958. Cînepa - Studiu monografic. [Hemp - monographic study.] Ed. Acad. R.P.R. Bucharest, Romania. [Translation I. Bócsa.]
Clarke, R. C. 1997. Hanf - Botanik, Anbau, Vermehrung und Züchtung. [Hemp - botany, cultivation, propagation and breeding.] AT Verlag, Aarau, Switzerland. [in German]
de Meijer, E. P. M. 1995. Fiber hemp cultivars: A survey of origin, ancestry, availability and brief agronomic characteristics. Journal of the International Hemp Association 2(2): 66-73.
de Meijer, E. P. M. and L. C. P. Keizer 1994. Variation of Cannabis for phenological development and stem elongation in relation to stem production. Field Crops Research 38: 37-46.
de Meijer, E. P. M., H. J. van der Kamp and F. A. van Eeuwijk 1992. Characterisation of Cannabis accessions with regard to cannabinoid content in relation to other plant characters. Euphytica 62: 187-200.
European Union 1998. Ordinance (EG), 1420/98.
Hanway, J. J. 1963. Growth stages of corn (Zea mays L.). Agronomy Journal 55: 487-492.
Hennink, S. 1997. EU regulations on hemp cultivation. Journal of the International Hemp Association 4(1): 38-9.
Jefferies, R. A. and H. M. Lawson 1991. A key for the stages of development of potato (Solanum tuberosum). Annals of Applied Biology 119: 387-399.
Knott, C. M. 1987. A key for stages of development of the pea (Pisum sativum). Annals of Applied Biology 111:
Knott, C. M. 1990. A key for stages of development of the faba bean (Vicia faba). Annals of Applied Biology 116: 391-404.
McPartland, J. M. 1996a. A review of Cannabis diseases. Journal of the International Hemp Association 3(1): 19-23.
McPartland, J. M. 1996b Cannabis pests. Journal of the International Hemp Association 3(2): 49,52-5.
Mediavilla, V., P. Bassetti, M. Konermann and I. Schmid-Slembrouck 1998. Optimierung der Stickstoffdüngung und Saatmenge im Hanfanbau. [Optimizing nitrogen fertilization an seed density in hemp cultivation.] Agrarforschung 5(5): 241-244. [in German]
Meier, Ch. and V. Mediavilla 1998. Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil. Journal of the International Hemp Association 5(1): 16-20.
Slembrouck, I. 1994. Anbau von Hanf: Ertragsbildung unter verschiedenen klimatischen Bedingungen. [Hemp cultivation - yield components on different climatic situations.] Swiss Federal Institute of Technology, Department of Agriculture and Food Science, Institute of Plant Sciences, Zurich, Switzerland. [in German]
Sylvester-Bradley, R., R. J. Makepeace and H. Broad 1984. A code for stage development in oilseed rape (Brassica napus L.) - Agronomy, physiology, plant breeding and crop protection of oilseed rape. Aspects of Applied Biology 6: 399-419.
van der Werf, H. M. G., K. Brouwer, M. Wijlhuizen and J. C. M. Withagen 1995a. The effect of temperature on leaf appearance and canopy establishment in fiber hemp (Cannabis sativa L.). Annals of Applied Biology 126: 551-561.
van der Werf, H. M. G., M. Wijlhuizen and J. A. A. de Schutter 1995b. Plant density and self-thinning affect yield and quality of fiber hemp (Cannabis sativa L). Field Crops Research 40(3): 153-64.
von Buttlar, H.-B., F. Höppner, U. Menge-Hartmann, K. Scheffer and B. Mispelhorn 1997. Europäische Hanfsorten im Standortvergleich zweier deutscher Anbauregionen. [European hemp varieties compared on two different German regions.] nova Institut (Eds.) Bioresource hemp ‘97 Proceedings of the Symposium Frankfurt am Main Feb. 27th - Mar. 2nd 1997, First edition: 209-219. [in German]
Zadoks, J. C., T. T. Chang and C. F. Konzak 1974. A decimal code for the grown stages of cereals. Weed Research 14: 415-421.