4. The Rewarding System

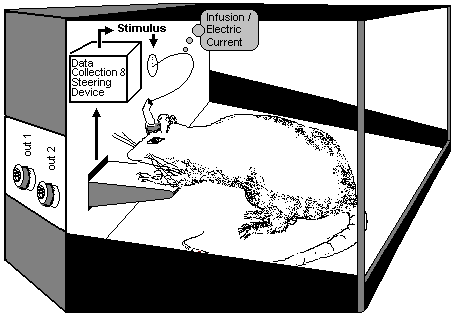
Fig.21: Self-Stimulation Apparatus
according to Olds (1976): The Switch of the Rewarding System.
The animal can release a reward in the form of either an electrical stimulation of the
rewarding system or a local application of an euphorising pharmacon by activating a
switch.
The rewarding system is demonstrated with the Olds’ Self stimulation experiment in Fig.21: electrodes are implanted at certain places in the forebrain of the animal. The animal can cause a stimulus by activating a switch. If the electrodes have been well-placed, the animal cannot be prevented from activating this switch. Even when a hot-plate is placed under the switch does not prevent the animal from stimulating the electrode. The stimulus on the rewarding system overrides the pain stimuli. The animal even neglect drinking and eating. The self-stimulation experiment represents the Physiological Addiction Model (Summary in Stolerman 1992). The rewarding system can also be stimulated through external influx of opioids or other dependency-causing pharmaca. The drugs can be applied into defined regions of the brain through catheter, intrathecal or locally in the self-stimuli experiment.
4.1 The Rewarding System as a Behaviour Model
The measured biological behavioural observations were conducted by Iwan Petrowitsch Pawlow (Nobelpreisträger von 1904) with his experiments with dogs. In his classic conditioning experiments, the animals learnt to react to one another with a certain reflex to certain stimuli. If a dog is presented with some food, a saliva secretion is released which is an unconditional Reflex. If a dog in training is presented with a food after the sound of a bell, the same reaction is released even when no food is given after ringing the bell. The classic Pawlow’sche conditioning continued independently of Rewarding- or Aversive-Stimuli. (see Tab.12).
The connection between the behavioural observations of animals and their functional anatomy was first demonstrated by the Zürcher Physiologe Walter Rudolf Hess (Nobelpreisträger von 1949), with localised, electrical stimuli experiments. Behavioural expressions such as fear, thirst, hunger, aggression and sexuality were observed by stimulating certain points in the hypothalamus.
The Rewarding system is in the behaviour model a control circuit with feed-back from stimuli an behaviour. B. F. Skinner used (1938) food, in his classic behavioural trial arrangement, as a reward and made operante Conditioning induced behaviour visible and easy to measure. The animal learnt to adapt it’s behaviour to operante strengthening stimuli, through trial and error (Trial and Error according to A. Bain 1855) (reward or aversion). In Olds (1976) self-stimuli apparatus, an applied electrical stimulation was used, through electrodes at certain points on the central nervous system (Nucleus accumbens, Ventral Tegmentum, Hypothalamus etc.) to positively stimulate.
Pleasant stimuli are positive reinforcers in self stimulation experiments. The most basic needs like eating, drinking or sex are mainly positively connected stimuli. What we enjoy doing, we like to do again.
Dependency-causing drugs work as positive reinforcers in self-stimulation experiments, as a reward: this applies to all opioid agonists, cocaine, for many amphetamines, Ecstasy, Barbiturates, Benzodiazepine, Alcohol, Nicotine and certain inhalable solubles. Rarely does a positive feedback occur with Cannabis THC and LSD tends to have a negative effect. Nicotine can be more negative under certain conditions: animals activate a switch in order to escape from programmed, not self-steered, nicotine infusions. Nicotine can even have the effect of a punishing stimuli: if the activation of a switch activates food dispension as well as an amount of nicotine, the tendency to switch decreases.
Behaviour can be crucially influenced with stimuli, Key-Stimuli, associated with drugs such as taste, smell or colour. Addictive drugs in self-stimuli experiment but also as a key-stimuli have an effect (discriminative stimulus): an animal is offered two switches to choose from. Tone switch administers a rewarding treat, if activated after administration of drug; the other rewards with a treat after administration of a placebo. The animals learn very fast, to differentiate between the placebo and the drug as a key-stimuli, in order to activate the right switch. The similarities or differences of drugs as key-stimuli can be measured in self-stimuli experiments. Most drugs can be very easily differentiated from one another. This property of subjective recognisability of drugs can effect the addiction in a strengthening way (sometimes also numbing). The positive feedback of the primary stimuli of a drug is through the effect of the key-stimuli, increased.
Animals learn, that opioids are administered after activating a switch together with a secondary stimuli (for example a lamp lights up). When the primary stimuli, in the form of opioids, is not administered, they let themselves be rewarded over a long period of time, through the secondary stimuli and they keep pressing the switch until the light lights up. Conditional stimuli are, as situated influences on the environment, obviously part of the maintenance of and in fall backs in an addictive behaviour.
Effects of opioids in the rewarding system can be represented and measured in three different orders:
4.2 The Rewarding system and it’s Transmitters
The rewarding system is based on nerve cells with different transmitters and types of receptors.
Dopamine (DA) plays a central part in the rewarding system. Dopamine is generally an important substance for nerve cell metabolism and one of the most important transmitters in the CNS. The transmitter Noradrenalin (NA) can be synthesised out of dopamine.
Cells containing dopamine can be selectively and irreversibly destroyed with 6-hydroxy-Dopamine. Anatomical and functional consequences can be observed as a result. Neuroleptica reversibly block the dopamine receptors. Due to this blocking, different Dopamine receptor-Subtypes (D1 - D5) were found.
Two important dopamine systems are rooted in the middle brain: the nigrostriatal path (the extrapyramidal Motorik, sensitive movements) and the mesolimbic System (mesolymbic dopamine system). A small dopamine path, the tubero-infundibule System arises from the Nucleus arcuatus of the hypothalamic Infundibulums, goes into the Hypophyse (Gland hanging on the brain) and inhibits, here, the secretion of Prolactin.
The mesolimbis Dopamine system increases the drive and is modulated in an inhibiting fashion through the neurotransmitter GABA (g -Amino-Butter-Acid), Noradrenalin and endogenic opioid peptides.
4.3 The Path of Dopamine from the Midbrain to the Basis of the Forebrain
The cell body of the mesolimbic System lies in the ventral tegmental area (VTA) of the middle brain and projects into the following structures of the front brain: Nucleus accumbens, Tuberculum olfactorium, frontal Cortex, cingular Cortex and Amygdala. These projections of the mesolimbic dopamine path correspond to the parts of the limbic system, which are connected to emotions. The limbic System is responsible for the ability to learn, the short-term memory, maintenance of moods and the alignment of thought and action.
The Nucleus accumbens, both sides of the septum at the base of the entorhinal Cortex, is the most important end point of the mesolimbic dopamine path. Nerve fibres project out of the Nucleus accumbens mainly into the cortex and into the extrapyramidalmotoric system.
The mesolimbic system represents a modulation system, somehow a filter and gating system for signals coming from the limbic system and for signals which maintain the basic functions of life and motivation (Koob 1992). The Nucleus accumbens, is somewhat of a clearing centre for signals coming from the limbic system and the arousal system. The Nucleus accumbens is the switching point, which anticipates the probability of reward-stimuli and steers the attention of the nerve system.
4.4 The Rewarding System and Addictive Drugs
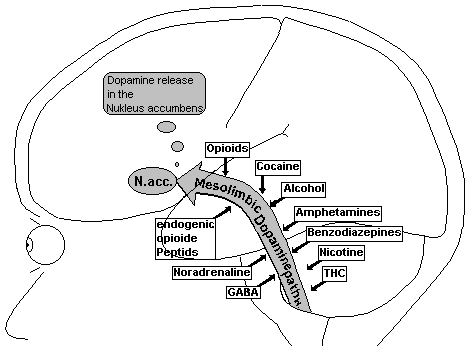
Fig.22: Mesolimbic Dopamine Pathway
All potentially addictive Pharmaca release positive boosts and seems to be able to modulate the mesolimbic system. One can prove the release of dopamine from the nucleus accumbens through mikrodialysis as a consequence of administering potentially addictive drugs. Cannabis THC, despite causing the release of Dopamine in Nucleus accumbens, but it does not release a positive boost in the self-stimuli experiment.
The potentially addictive effects of Amphetamines (‘Speed’, ‘Ecstasy’) and Cocaine appear to dependent on the path of dopamine of the mesolimbic system (Koob 1992). Benzodiazepine take effect on the GABA-receptors on the mesolimbic dopamine system. Certain dopamine receptors in the Nucleus accumbens are activated through administration of Alcohol or Nicotine. It was recently proven that, Cannabis D 9-Tetrahydro-Cannabinol (THC, effective substance in Marihuana and Hash) can cause releases of dopamine in the Nucleus accumbens, which are suppressed by the Opioid antagonist, Naloxon (Chen 1989).
Opioids can also activate the mesolimbic dopamine system and important connections concerning opioid-dependency can be explained by this. Opioids seem to boost the release of dopamine in the Nucleus accumbens through the m -receptors-carrying neurones by inhibiting GABA interneurones; this is an inhibition of an inhibition. The d -receptors in connection with D1-receptors also play a role in the mesolimbic system. The function of the d -receptors is unclear since is was shown that no reward-effects were found in cloned mice, without m -receptors (Matthes 1996). k -neurone directly inhibit the mesolimbic path of dopamine in the and the release of dopamine in the Nucleus accumbens, which could explain the Dysphoria through k -receptor stimulation.
Tab.13: Effects and localisation of the three Opioid-receptor-subtypes (according to Mansour 1995)
Reward-effects of opioids are found at other sites on the CNS. m -receptors transmit reward-stimuli in the mesolimbic system, in the Hippocampus as well as in the Hypothalamus.
Opioids can act as reward-stimuli m -receptors, independent of the dopamine system. Dopamine-independent positive boosts have been seen in opioids as well as in Alcohol (Koob 1992).
4.5 Interpretation of the Rewarding System and Addiction
The drugs, which the animals take themselves in the self-stimulation experiments, are without exception, potentially highly addictive for humans (Koob 1992). Addictive drugs are replacements for the natural rewarding-stimuli. Addictive drugs and natural rewarding-stimuli show similar, basic properties in influencing the transmission of dopamine (Di Chiara 1992).
All positive stimuli are transmitted through the Rewarding system: contentment after eating, drinking, love, solving a problem and possibly every orientation of our wishes and our actions have some connection with this Rewarding system . In the CNS , all the nerve cells are connected to one another by few in-between-stops (‘omniconnected brain’ Pribram). In the thoughts of the animals in the self-stimuli experiment, it is guessed that every activity in the large brain seeks and finds a way to the ‘switch’ of the Rewarding system. That way, no thoughts can get past the Rewarding system.
A type of verification in relation to vital functions, is conducted for each nerve activity, in the Rewarding system. The permittance or ‘truth’ of every memory, every action or every thought occurring in the brain due to this motivating examination, gives to what extent the rewarding-system is then stimulated. Every product, whether it is totally primitive or highly abstract, (man’s) large brain becomes initially a unit structure and the action of a living form. Apparently, a united thinking and action is only then permissible after the verification process of the rewarding system is complete.
Electrostimulation on the Rewarding system (Olds’scher Self-Stimuli experiment), systematic of local drug intake, sex magazines, TV, food with or without sugar, prepared foods, thrills when Bungee Jumping, Jogging and many others belong to the ever increasing list of human possibilities to avoid the natural permittance of the rewarding systems. Addiction can occur, in the logic of the physiological Addiction Model, when shortcuts to the inner ‘switch’ of the Rewarding system can be found. The higher the probability is that a positive boost is released from the stimulus of an addictive substance, the less likely it is that other stimuli can influence the behaviour of an individual; the simple ‘permittance to the switch then takes upperhand over nature. The prevention and overcoming from an addictive behaviour requires the training and maintenance of many different possibilities to stimulate the Rewarding systems. Negative boosting stimuli and punishing stimuli have a noticeably less effect on the addictive behaviour in animal experiments as the positive boosting stimuli, of addictive substances, do.